
- 8.1. Clinical Trial
- 8.1.1. Ovarian
- 8.1.2. Endometrial
- 8.2. Supportive Cancer Care
- 8.3. Data Science
- 8.4. Translational Research
- 8.5. Basic Research
|
8. Gynae-oncology |
|
||||||||||
|
8.1 Clinical Trial |
|
||||||||||
|
8.1.1.1 Ovarian |
|
||||||||||
|
Stage 3 or 4 ovarian, fallopian tube or primary peritoneal carcinoma |
|
||||||||||
|
Specific Selection Criteria: Previous treatment response to neoadjuvant treatment |
|
||||||||||
|
Neoadjuvant (Maintenance) treatment: Phase 2 |
Targeted Therapy (PARP Inhibitor) |
|
|||||||||
|
Study Title |
Main Inclusion/Exclusion |
Investigational Product |
Principal Investigator |
Department |
|
Contact number |
|
||||
|
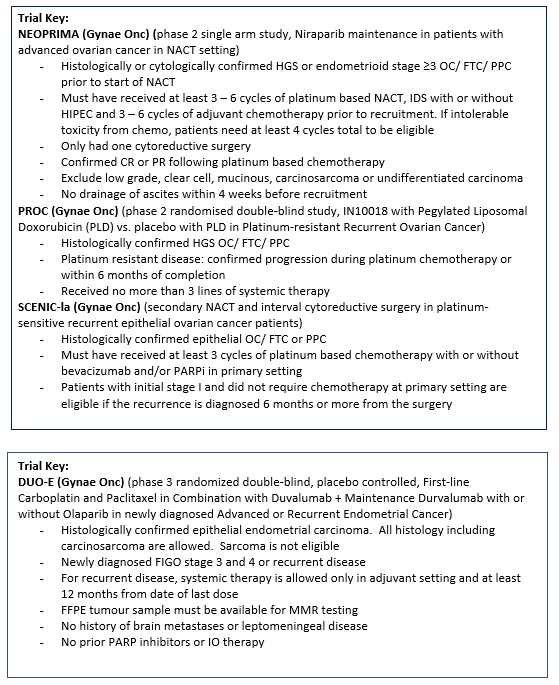
Niraparib maintenance in patients with advanced ovarian cancer at neoadjuvant setting - a phase 2, single-arm trial (NEOPRIMA trial). |
Key Inclusion Criteria:
Key Exclusion Criteria:
|
Niraparib (PARP Inhibitor) |
Dr. Ka Yu Tse |
Department of Obstetrics & Gynaecology
|
tseky@hku.hk |
Dr. Ka Yu Tse 2255 5102 9061 9609 |
|
||||
|
8.1.1.2 Ovarian |
|
||||||||||
|
Recurrent |
|
||||||||||
|
Specific Selection Criteria: Received platinum containing treatment and progressed |
|
||||||||||
|
Second to Fourth-line treatment: Phase 2 |
Targeted Therapy (FAK Inhibitor), Chemotherapy |
|
|||||||||
|
A Multicenter, Randomized, Double-Blind, Phase II Clinical Study of IN10018 in Combination with Pegylated Liposomal Doxorubicin (PLD) vs. Placebo in Combination with PLD for the Treatment of Platinum-resistant Recurrent Ovarian Cancer. |
Key Inclusion Criteria:
|
IN10018 (FAK inhibitor) + Pegylated Liposomal Doxorubicin (Chemo)
Vs
Placebo + Pegylated Liposomal Doxorubicin |
Dr. Ka Yu Tse |
Department of Obstetrics & Gynaecology
|
tseky@hku.hk |
Dr. Ka Yu Tse 2255 5102 9061 9609 |
|
||||
|
8.1 Clinical Trial |
|
||||||||||
|
8.1.2.1 Endometrial |
|
||||||||||
|
Advanced or Recurrent |
|
||||||||||
|
First-line treatment: Phase 3 |
Immunotherapy (Anti-PD-L1), Targeted Therapy (PARP inhibitor), Chemotherapy |
|
|||||||||
|
Study Title |
Main Inclusion/Exclusion |
Investigational Product |
Principal Investigator |
Department |
|
Contact number |
|||||
|
DUO-E (Protocol ID: D9311C00001): A Randomised, Multicentre, Double-blind, Placebo-controlled, Phase III Study of First-line Carboplatin and Paclitaxel in Combination with Duvalumab, Followed by Maintenance Durvalumab with or without Olaparib in Patients with Newly Diagnosed Advanced or Recurrent Endometrial Cancer.
|
Key Inclusion Criteria:
|
carboplatin + paclitaxel + durvalumab (Anti-PD-L1) + Maintenance: durvalumb + Olaparib (PARP inhibitor)/ Placebo
Vs
carboplatin + paclitaxel + Placebo + Maintenance: Placebo |
Dr. Karen Chan |
Department of Obstetrics & Gynaecology |
obsgyn@hku.hk |
2255 4265
|
|||||
|
8.0 Gynae-oncology |
|
||||||||||
|
8.3.1 Data Science |
|
||||||||||
|
Study Title |
Main Inclusion/Exclusion |
Principal Investigator |
Department |
|
Contact number |
|
|||||
|
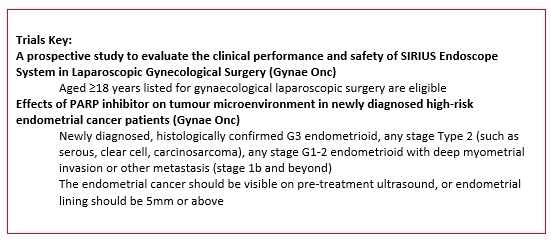
A prospective study to evaluate the clinical performance and safety of SIRIUS Endoscope System in Laparoscopic Gynecological Surgery. |
Key Inclusion Criteria:
Key Exclusion Criteria:
|
Dr. Siew Fei Ngu |
Department of Obstetrics and Gynaecology |
ngusiewf@hku.hk |
Dr. Siew Fei Ngu 2255 4265 |
|
|||||
|
8.0 Gynae-oncology |
|
||||||||||
|
8.4.1 Translational Research |
|
||||||||||
|
Study Title |
Main Inclusion/Exclusion |
Principal Investigator |
Department |
|
Contact number |
|
|||||
|
Effects of PARP inhibitor on tumour microenvironment in high-risk endometrial cancer patients. |
Key Inclusion Criteria:
|
Dr. Tse Ka Yu |
Department of Obstetrics and Gynaecology |
tseky@hku.hk |
Dr. Ka Yu Tse 2255 5102 9061 9609 |
|
|||||
|
8.4.2 Translational Research |
|||||
|
Study Title |
Main Inclusion/Exclusion |
Principal Investigator |
Department |
|
Contact number |
|
Secondary neoadjuvant chemotherapy and interval cytoreductive surgery in platinum-sensitive recurrent epithelial ovarian cancer patients – a pilot study (SCENIC-Ia study). |
Key Inclusion Criteria:
|
Dr. Tse Ka Yu |
Department of Obstetrics and Gynaecology |
tseky@hku.hk |
Dr. Ka Yu Tse 2255 5102 9061 9609 |
|
8.0 Gynae-oncology |
||||
|
8.5.1 Basic Research |
||||
|
Study Title |
Principal Investigator |
Department |
|
Contact number |
|
Elucidating the cooperative role of PIK3CA and ADAM15 in serous ovarian carcinoma. |
Dr Lydia Wai Ting Cheung |
School of Biomedical Sciences
|
lydiacwt@hku.hk |
Dr Lydia Wai Ting Cheung 3917 6908 |
|
8.5.2 Basic Research |
||||
|
Elucidation of a novel p85β-AXL regulatory loop and associated signaling in ovarian cancer. |
Dr Lydia Wai Ting Cheung |
School of Biomedical Sciences
|
lydiacwt@hku.hk |
Dr Lydia Wai Ting Cheung 3917 6908 |
|
8.5.3 Basic Research |
||||
|
Precision medicine strategies for ovarian cancer |
Dr Lydia Wai Ting Cheung |
School of Biomedical Sciences
|
lydiacwt@hku.hk |
Dr Lydia Wai Ting Cheung 3917 6908 |
|
8.5.4 Basic Research |
||||
|
PIK3R2 amplification in ovarian cancer: functional significance and implication for cancer therapy. |
Dr Lydia Wai Ting Cheung |
School of Biomedical Sciences |
lydiacwt@hku.hk |
Dr Lydia Wai Ting Cheung 3917 6908 |
|
8.5.5 Basic Research |
||||
|
Systematic identification of “BRCAness” in ovarian cancer |
Dr Lydia Wai Ting Cheung |
School of Biomedical Sciences |
lydiacwt@hku.hk |
Dr Lydia Wai Ting Cheung 3917 6908 |
|
8.5.6 Basic Research |
||||
|
Understanding the role of intrinsic type 1 interferon pathway in bTCRP1-mediated ovarian cancer chemoresistance |
Dr Lydia Wai Ting Cheung |
School of Biomedical Sciences |
lydiacwt@hku.hk |
Dr Lydia Wai Ting Cheung 3917 6908 |
|
8.5.7 Basic Research |
||||
|
Targeting PIK3R1 copy number loss in ovarian cancer |
Dr Lydia Wai Ting Cheung |
School of Biomedical Sciences |
lydiacwt@hku.hk |
Dr Lydia Wai Ting Cheung 3917 6908 |

Follow HKUMed