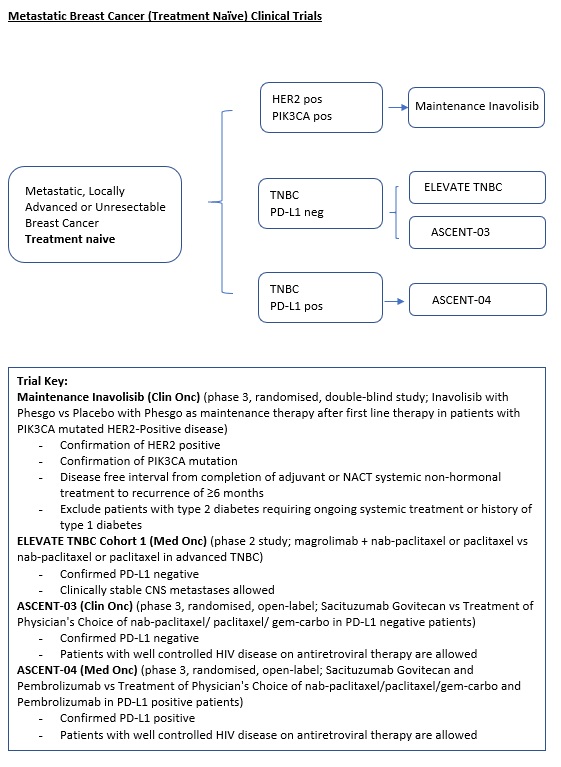
- 3.1. Clinical Trial
- 3.1.1. Triple Negative (TNBC)
- 3.1.1.1. Early Stage
- 3.1.1.2. Advanced Stage
- 3.1.2. HER2+ve
- 3.1.2.1. Early Stage
- 3.1.2.2. Advanced Stage
- 3.1.3. ER+ve & HER2-ve
- 3.1.3.1. Early Stage
- 3.1.3.2. Advanced Stage
- 3.1.1. Triple Negative (TNBC)
- 3.2. Supportive Cancer Care
- 3.3. Data Science
- 3.4. Basic Research
3.0 Breast |
||||||
|
3.1 Clinical Trial |
||||||
|
3.1.1 Triple Negative (TNBC) |
||||||
|
3.1.1.1.1 Early Stage |
||||||
|
Specific Selection Criteria: Glob H-Positive |
||||||
|
Adjuvant treatment: Phase 3 |
Immunotherapy (Globo H-KLH immunostimulant) |
|||||
|
Study Title |
Main Inclusion/Exclusion |
Investigational Product |
Principal Investigator |
Department |
|
Contact number |
|
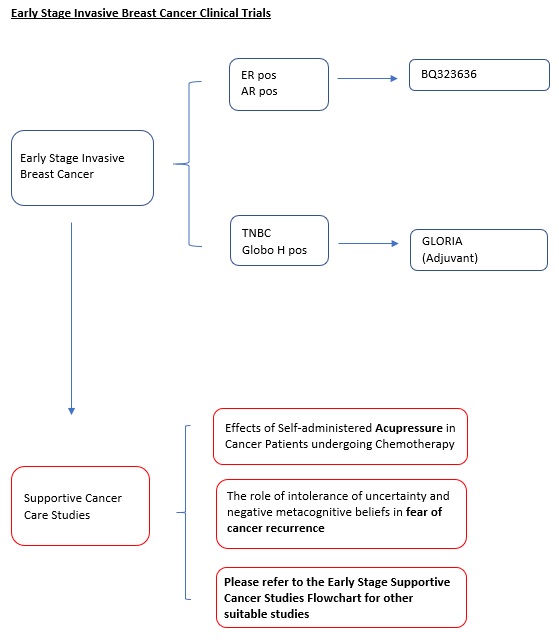
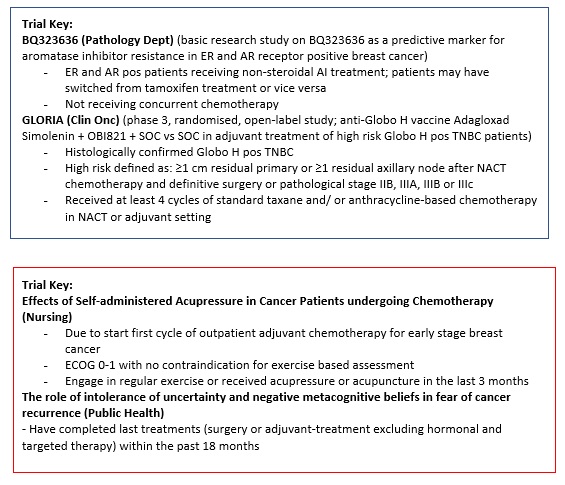
The GLORIA Study (Protocol ID: OBI-822-011): A Phase 3, Randomized, Open-Label Study of the Anti-Globo H Vaccine Adagloxad Simolenin (OBI-822)/OBI-821 in the Adjuvant Treatment of Patients with High-Risk, Early-Stage Globo H-Positive Triple Negative Breast Cancer. |
Key Inclusion Criteria:
|
Adagloxad Simolenin (Globo H-KLH immunostimulant) + OBI-821
Vs.
SOC. |
Dr. Wendy CHAN |
Department of Clinical Oncology
|
winglok@hku.hk |
Alex Mak 2255 4216 |
|
3.1.1 Triple Negative (TNBC) |
||||||
|
3.1.1.2.1 Locally Advanced or Metastatic, Unresectable |
||||||
|
Specific Selection Criteria: PD-L1-ve (Cohort 1), PD-L1+ve (Cohort 2) |
||||||
|
First-line (Cohort 1), Second to Third-line (Cohort 2) treatment: Phase 2 |
Immunotherapy (Anti-CD47 Antibody) |
|||||
|
Study Title |
Main Inclusion/Exclusion |
Investigational Product |
Principal Investigator |
Department |
|
Contact number |
|
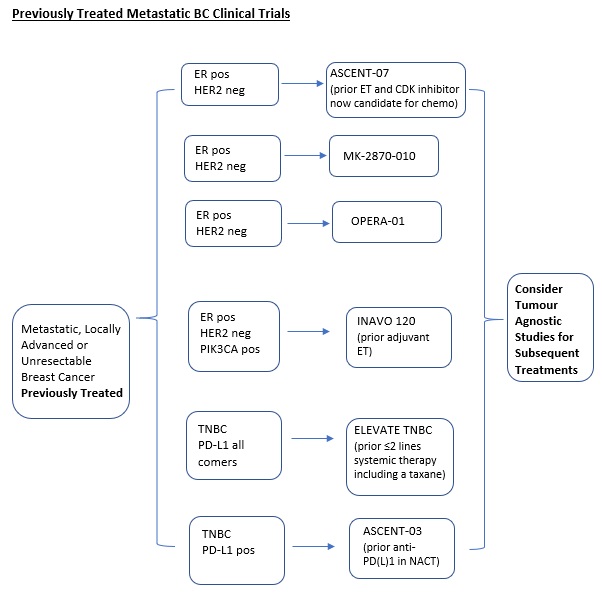
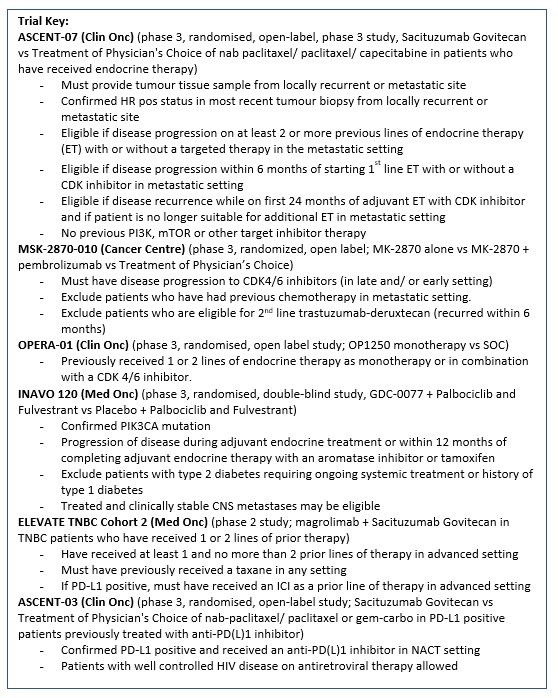
A phase 2 study of magrolimab combination therapy in patients with unresectable, locally advanced or metastatic triple-negative breast cancer. |
Key Inclusion criteria:
|
Magrolimab (Anti-CD47 Monoclonal Antibody) + Nab-Paclitaxel or Paclitaxel (Chemo)
Vs.
Nab-Paclitaxel or Paclitaxel (Chemo)
|
Dr. Joanne Chiu |
Department of Medicine (Medical Oncology) |
jwychiu@hku.hk |
2255 5582 |
|
3.1.1 Triple Negative (TNBC) |
||||||
|
3.1.1.2.2 Locally advanced or Metastatic, Unresectable |
||||||
|
First-line treatment: Phase 3 |
Targeted Therapy (Anti-Trop-2 ADC), Immunotherapy (Anti-PD-1 Antibody) |
|||||
|
Study Title |
Main Inclusion/Exclusion |
Investigational Product |
Principal Investigator |
Department |
|
Contact number |
|
ASCENT-04 (Protocol ID: GS-US-592-6173): A Randomized, Open-label, Phase 3 Study of Sacituzumab Govitecan and Pembrolizumab Versus Treatment of Physician's Choice and Pembrolizumab in Patients With Previously Untreated, Locally Advanced Inoperable or Metastatic Triple-Negative Breast Cancer, Whose Tumors Express PD-L1. |
Key Inclusion Criteria:
|
Sacituzumab govitecan-hziy (SG) (Antibody drug conjugate) + Pembrolizumab (Anti-PD-1 monoclonal Antibody)
Vs.
Pembrolizumab (Anti-PD-1 monoclonal Antibody) + Treatment of Physician's Choice (TPC) |
Dr. Joanne Chiu |
Department of Medicine (Medical Oncology) |
jwychiu@hku.hk |
2255 5582 |
|
3.1.1 Triple Negative (TNBC) |
||||||
|
3.1.1.2.3 Locally Advanced or Metastatic, Unresectable |
||||||
|
Specific Selection Criteria: PD-L1-ve (For Untreated), PD-L1+ve (For Previously Treated with Anti-PD-L1 Agents) |
||||||
|
First-line and Second-line treatment: Phase 3 |
Targeted Therapy (Anti-Trop-2 ADC) |
|||||
|
Study Title |
Main Inclusion/Exclusion |
Investigational Product |
Principal Investigator |
Department |
|
Contact number |
|
ASCENT-03 (Protocol ID: GS-US-592-6238): A Randomized, Open-label, Phase 3 Study of Sacituzumab Govitecan Versus Treatment of Physician’s Choice in Patients With Previously Untreated, Locally Advanced, Inoperable or Metastatic Triple-Negative Breast Cancer Whose Tumors Do Not Express PD-L1 or in Patients Previously Treated with Anti-PD-(L)1 Agents in the Early Setting Whose Tumors Do Express PD-L1. |
Key Inclusion criteria:
|
Sacituzumab Govitecan (Anti-Trop-2-ADC)
Vs.
Paclitaxel/nab-Paclitaxel/ Gemcitabine (Chemo) + Carboplatin (Chemo)
|
Dr. Wendy Chan |
Department of Clinical Oncology |
winglok@hku.hk |
Kary Yeung 2255 5124 |
|
3.1.2 HER2+ve |
||||||
|
3.1.2.2 Locally Advanced or Metastatic |
||||||
|
Specific Selection Criteria: PIK3CA-Mutated HER2+ve, after first-line induction therapy |
||||||
|
Maintenance Therapy: Phase 3 |
Targeted Therapy (PI3Kα Inhibitor/ Anti-HER2 antibody) |
|||||
|
Study Title |
Main Inclusion/Exclusion |
Investigational Product |
Principal Investigator |
Department |
|
Contact number |
|
A Phase III, Multicenter, Randomized, Double-Blind, Placebo-Controlled Study Evaluating the Efficacy and Safety of Inavolisib in Combination With Phesgo Versus Placebo in Combination With Phesgo As Maintenance Therapy After First Line Induction Therapy in Participants With PIK3CA-Mutated HER2-Positive Locally Advanced or Metastatic Breast Cancer. |
Key Inclusion Criteria:
|
Inavolsib (PI3Kα Inhibitor) + Phesgo (Anti-HER2 antibody)
Vs
Placebo + Phesgo |
Dr. Wendy Chan |
Department of Clinical Oncology
|
winglok@hku.hk |
Emina CHEUNG 2255 5124 |
|
3.1.3 ER+ve, HER2-ve |
||||||
|
3.1.3.2.1 Locally Advanced or Metastatic, Unresectable |
||||||
|
Second-line treatment: Phase 3 |
Targeted Therapy (PI3K inhibitor/ CDK Inhibitor), Hormonal therapy (Estrogen receptor antagonist) |
|||||
|
Study Title |
Main Inclusion/Exclusion |
Investigational Product |
Principal Investigator |
Department |
|
Contact number |
|
INAVO120 (Protocol ID: WO41554): A Phase III, Randomized, Double-Blind, Placebo-controlled Study Evaluating the Efficacy and Safety of GDC-0077 Plus Palbociclib And Fulvestrant Versus Placebo Plus Palbociclub and Fulvestrant in Patients with PIK3CA-Mutant, Hormone Receptor-Positive, HER2-Negative Locally Advanced or Metastatic Breast Cancer. |
Key Inclusion Criteria:
|
GDC-0077 (PI3K inhibitor)
Vs.
Placebo (Co-admin: Palbociclib - Cyclin-dependent kinase (CDK) inhibitor, Fulvestrant - Estrogen receptor antagonist) |
Dr. Roland Leung |
Department of Medicine (Medical Oncology) |
medicaloncology@hku.hk |
2255 5582 |
|
3.1.3 ER+ve, HER2-ve |
||||||
|
3.1.3.2.2 Locally Advanced or Metastatic, Unresectable |
||||||
|
Second-line or Subsequent-line treatment: Phase 3 |
Targeted Therapy (Anti-Trop-2 ADC), Chemotherapy |
|||||
|
Study Title |
Main Inclusion/Exclusion |
Investigational Product |
Principal Investigator |
Department |
|
Contact number |
|
ASCENT-07 (Protocol ID: GS-US-598-6168): A Randomized, Open-label, Phase 3 Study of Sacituzumab Govitecan Versus Treatment of Physician's Choice in Patients With Hormone Receptor-Positive (HR+)/Human Epidermal Growth Factor Receptor 2 Negative (HER2-) (HER2 IHC0 or HER2-low [IHC 1+, IHC 2+/ISH-]) Inoperable, Locally Advanced, or Metastatic Breast Cancer and Have Received Endocrine Therapy. |
Key Inclusion Criteria:
|
Sacituzumab govitecan (Anti-Trop-2 ADC)
Vs
Paclitaxel/ nab-paclitaxel/ capecitabine |
Dr. Wendy Chan |
Department of Clinical Oncology
|
winglok@hku.hk |
Bonnie Lau 2255 5124 |
|
3.1.3 ER+ve, HER2-ve |
||||||
|
3.1.3.2.3 Locally Advanced or Metastatic, Unresectable |
||||||
|
Second-line or Third-line treatment: Phase 3 |
Hormonal therapy (Estrogen receptor antagonist) |
|||||
|
Study Title |
Main Inclusion/Exclusion |
Investigational Product |
Principal Investigator |
Department |
|
Contact number |
|
OPERA-01 (Protocol ID: OP-1250-301): A phase 3 randomized, open-label study of OP-1250 monotherapy vs standard of care for the treatment of ER+, HER2– advanced or metastatic breast cancer following endocrine and CDK 4/6 inhibitor therapy. |
Key Inclusion Criteria:
a CDK 4/6 inhibitor. |
OP-1250 monotherapy
vs.
Control Arm |
Dr. Wendy CHAN |
Department of Clinical Oncology
|
winglok@hku.hk |
Alex MAK 2255 4216 |
|
3.1.3 ER+ve, HER2-ve |
||||||
|
3.1.3.2.4 Locally Advanced or Metastatic, Unresectable |
||||||
|
Second-line treatment: Phase 3 |
Antibody-Drug Conjugate (TROP2) |
|||||
|
Study Title |
Main Inclusion/Exclusion |
Investigational Product |
Principal Investigator |
Department |
|
Contact number |
|
MK2870-10 An Open-label, Randomized Phase 3 Study of MK-2870 as a Single Agent and in Combination with Pembrolizumab Versus Treatment of Physician’s Choice in Participants with HR+/HER2- Unresectable Locally Advanced or Metastatic Breast Cancer |
Key Inclusion Criteria:
Key Exclusion Criteria:
|
MK2870
Vs.
MK2870 + Pembrolizumab
Vs.
Physician’s Choice: Paclitaxel / Nab-Paclitaxel / Capecitabine/ Liposomal Doxorubicin |
Prof. Rina Hui |
Centre of Cancer Medicine |
cancermed@hku.hk |
3910 3339 |
|
3.2.3 Supportive Cancer Care |
|
||||||||||
|
Early Stage |
|
||||||||||
|
Specific Selection Criteria: Scheduled to commence their first cycle of outpatient adjuvant chemotherapy |
|
||||||||||
|
Study Title |
Main Inclusion/Exclusion |
Principal Investigator |
Department |
|
Contact number |
|
|||||
|
Effects of Self-administered Acupressure in Cancer Patients undergoing Chemotherapy: A Randomized Controlled Trial. |
Key Inclusion Criteria:
Key Exclusion Criteria:
|
Dr. Denise Shuk Ting Cheung |
School of Nursing |
denisest@hku.hk |
3917 6673 |
|
|||||
3.0 Breast |
|||||||||||
|
3.3.1 Data Science |
|||||||||||
|
Specific Selection Criteria: Early stage, completed the last treatments within the past 18 months |
|||||||||||
|
Study Title |
Main Inclusion/Exclusion |
Principal Investigator |
Department |
|
Contact number |
||||||
|
The role of intolerance of uncertainty and negative metacognitive beliefs in fear of cancer recurrence: a longitudinal study. |
Key Inclusion Criteria:
Key Exclusion Criteria:
|
Dr. Wendy WT Lam |
School of Public Health |
wwtlam@hku.hk |
3917 9878 |
||||||
|
3.0 Breast |
|||||||||||
|
3.4.1 Basic Research |
|||||||||||
|
Study Title |
Main Inclusion/Exclusion |
Principal Investigator |
Department |
|
Contact number |
||||||
|
Breast cancer risks following antipsychotic use in women with bipolar disorder versus schizophrenia: A territory-wide nested case-control study spanning two decades. |
Key Inclusion criteria:
|
Dr. Francisco T.T. Lai |
Department of Pharmacology and Pharmacy |
fttlai@hku.hk |
3910 3836 |
||||||
|
3.4.2 Basic Research |
|||||||||||
|
BQ323636.1 a predictive marker for aromatase inhibitor resistance in Estrogen and Androgen Receptor positive breast cancer. |
Key Inclusion Criteria:
Key Inclusion Criteria:
|
Prof. Ui Soon Khoo |
Department of Pathology |
uskhoo@hku.hk |
2255 2664 |
||||||
|
3.4.3 Basic Research |
|||||||||||
|
Functional characterization of AKTIP in breast cancer |
Dr. Lydia Wai Ting Cheung |
School of Biomedical Sciences |
lydiacwt@hku.hk |
Dr Lydia Wai Ting Cheung 3917 6908 |
|||||||
|
3.4.4 Basic Research |
|||||||||||
|
Pharmacological effect of Garcinone E on triple negative breast cancer. |
Dr. George P.H. Leung |
Department of Pharmacology and Pharmacy |
gphleung@hku.hk |
3917 6861 |
|||||||

Follow HKUMed