24 January 2024
An interdisciplinary research team led by Professor Jason Cheung Pui-yin from the Department of Orthopaedics and Traumatology, School of Clinical Medicine, LKS Faculty of Medicine of the University of Hong Kong (HKUMed); Dr Song You-qiang, School of Biomedical Sciences, HKUMed; and Dr Gao Bo, School of Biomedical Sciences, Faculty of Medicine, The Chinese University of Hong Kong; has achieved a global breakthrough by identifying a common underlying cause of adolescent idiopathic scoliosis (AIS), the most common form of spinal deformity, which affects millions of adolescents worldwide. The team discovered that variations in the SLC6A9 gene may cause idiopathic scoliosis, resulting in dysfunction of synaptic neurotransmission and central pattern generators (CPGs). This significant discovery was published in The Journal of Clinical Investigation [link to the publication].
Background
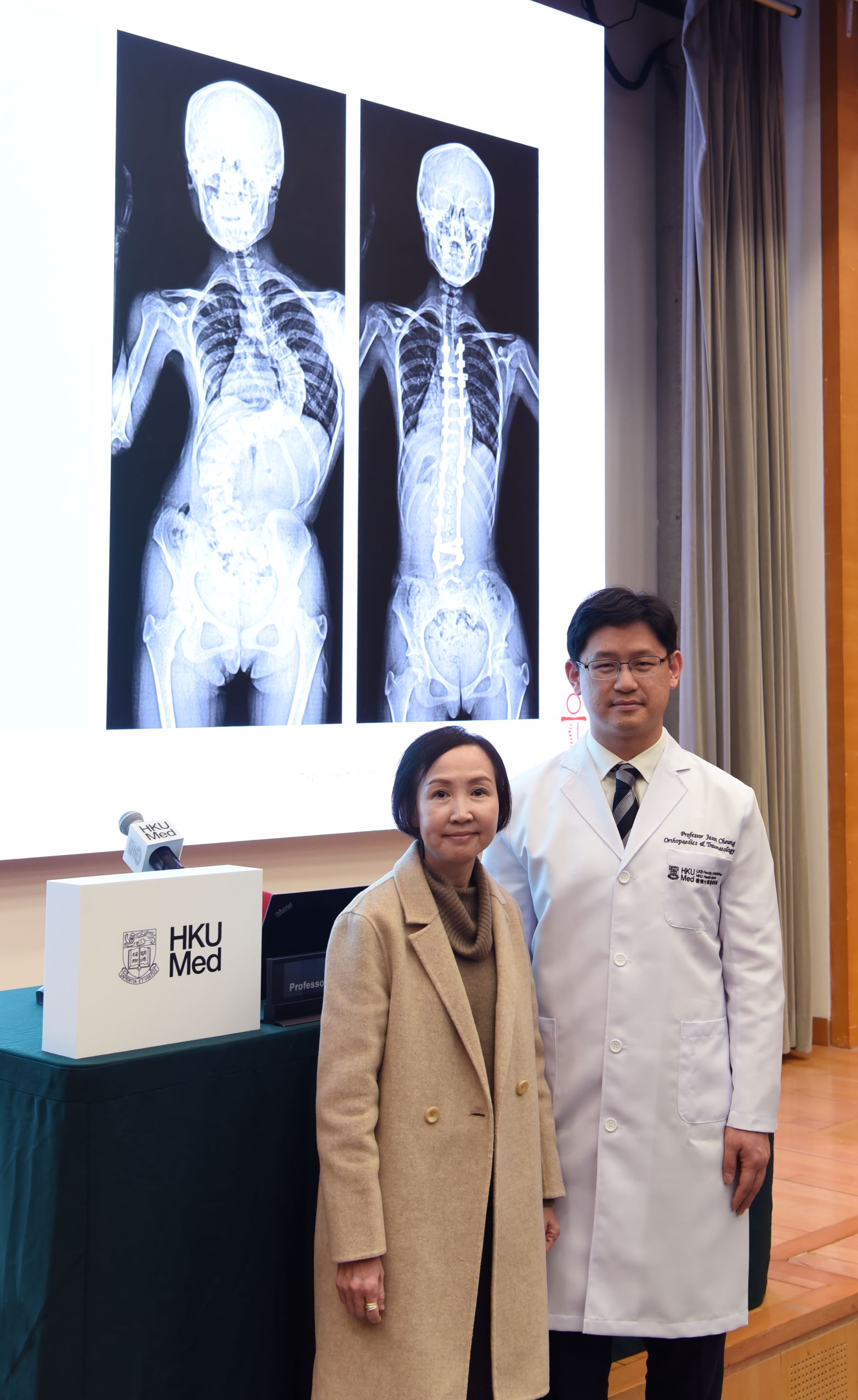
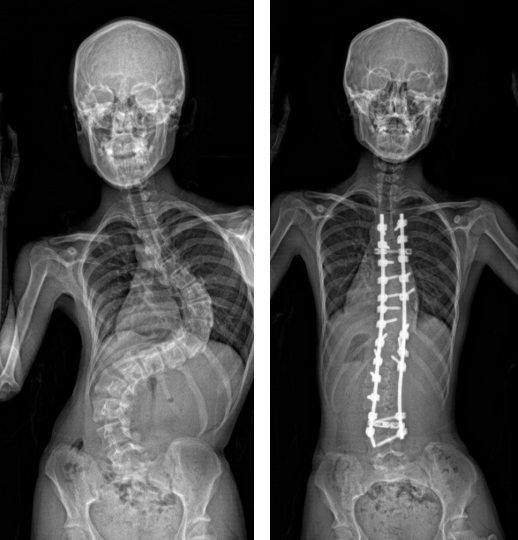
AIS is a common skeletal disorder, characterised by a lateral curvature of the spine exceeding 10 degrees, which affects 3.5% of adolescents aged between 10 and 18 in Hong Kong[1] . It worsens during puberty and can result in various complications including impaired cardiopulmonary functions, shortness of breath, back pain, uneven shoulders and hips, and overall poor cosmesis. These physical challenges can impact the social and psychological well-being of the affected individuals. Currently, treatment options are limited to bracing and invasive surgery after the condition develops. Preventive interventions remain unavailable, and its etiology is uncertain.
Research methods and findings
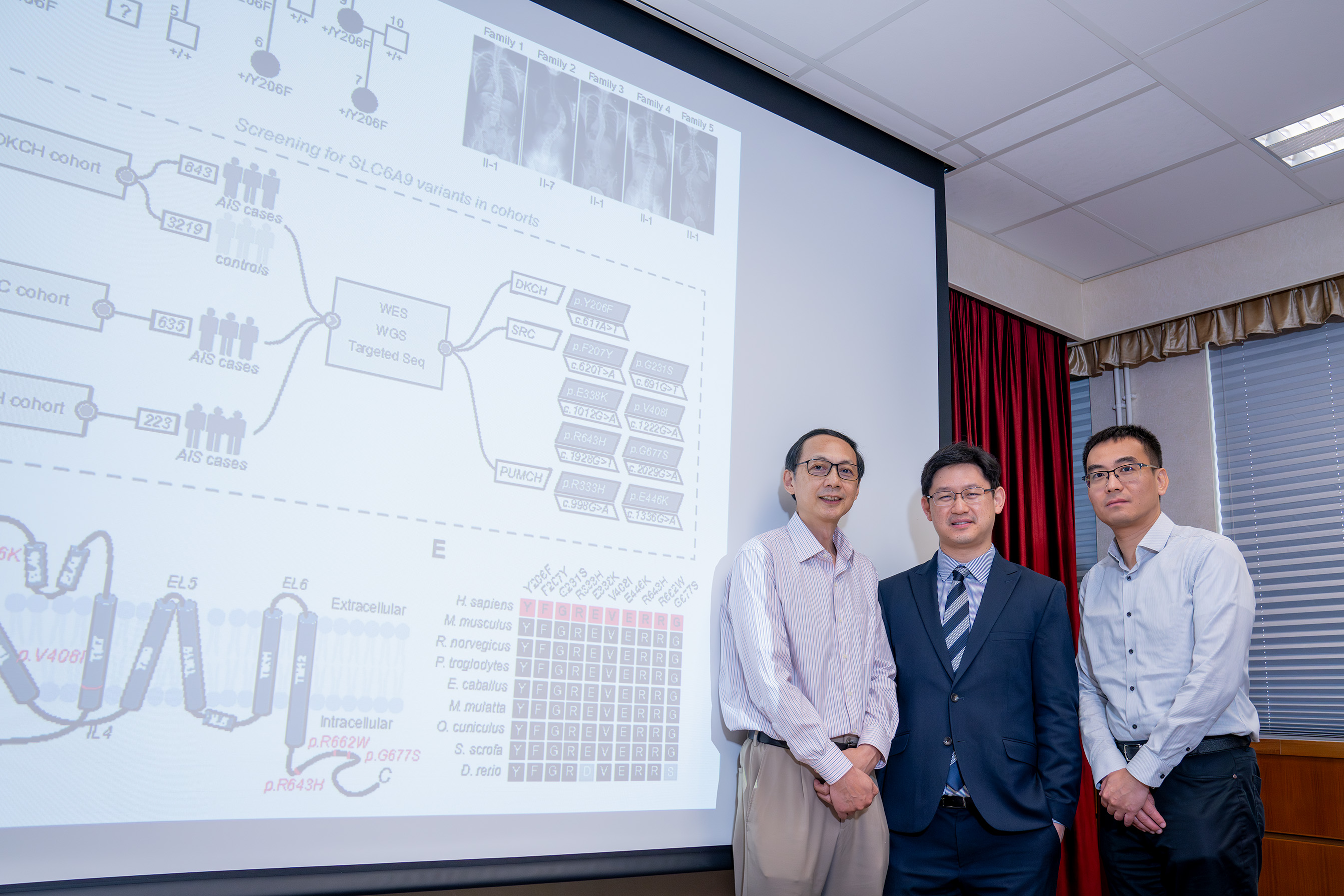
The research team investigated the genetic basis and pathogenic mechanism of AIS with a combined 1,701 AIS patients and 3,219 controls from the University of Hong Kong, Texas Scottish Rite Hospital for Children, and Peking Union Medical College Hospital in Beijing. They identified rare variants in the SLC6A9 gene, responsible for producing a protein called glycine transporter 1 (GLYT1), which can lead to disruptions in the transmission and coordination of nerve signals. GLYT1 plays a crucial role in regulating glycine levels in the body, which is essential for proper nerve signalling in the spine.
The team revealed that these identified variants in SLC6A9 resulted in reduced glycine uptake activity, leading to an increased glycine level and aberrant glycinergic neurotransmission. To further validate their findings, the team developed a novel zebrafish model with a disrupted SLC6A9 gene, mirroring the gene changes observed in humans with AIS. The mutant zebrafish exhibited spinal curvature and discoordination of spinal neural activity, resembling the symptoms found in AIS patients.
The team also investigated the role of CPGs, which are neural circuits in the spinal cord responsible for generating rhythmic movements, such as walking or breathing. They discovered that excessive glycine, caused by mutant GLYT1, may interfere with the normal function of CPGs, potentially contributing to the development of AIS.
In a further exploration of potential preventive therapies, the team tested sodium benzoate, a glycine neutraliser used clinically to treat patients with glycine encephalopathy, on the mutant zebrafish. The results showed that sodium benzoate can moderately reduce the severity of curvature in zebrafish, suggesting that it could provide a potential preventive therapy for AIS patients with high glycine levels.
AIS Cases
Among the patients involved in the study, Miss Lam and Miss Law were particularly instrumental in the success of the research. Both of them experienced severe scoliosis during their teenage years, and underwent surgical correction of their scoliosis deformities at the Duchess of Kent Children’s Hospital. Subsequently, 61% of Miss Lam’s family and 50% of Miss Law’s family also developed scoliosis, several of whom required surgical intervention. This SLC6A9 mutation carries an autosomal dominant pattern, which was found in the many involved members of their families.
Significance of the study
AIS lacks an agreed-upon etiology, impeding early intervention. While genetic factors are known to play a significant role in the development of AIS, the heritability and causal mechanisms remain unclear. ‘Our research efforts to identify AIS-related genetic variants can help fill the gaps, leading to targeted treatments and personalised medicine for AIS,’ said Professor Jason Cheung Pui-yin, Chairperson and Clinical Professor of the Department of Orthopaedics and Traumatology, School of Clinical Medicine, HKUMed.
This groundbreaking discovery by the HKUMed research team not only sheds light on the genetic factors contributing to AIS, but also paves the way for the development of new diagnostic methods and potential preventive therapies. ‘With further research and clinical trials, the findings may revolutionise the management of AIS and greatly improve the lives of adolescents affected by this condition,’ added Professor Cheung.
About the research team
The research was led by Professor Jason Cheung Pui-yin, Chairperson and Clinical Professor, Department of Orthopaedics and Traumatology, School of Clinical Medicine, HKUMed; Dr Song You-qiang, Principal Lecturer, School of Biomedical Sciences, HKUMed; and Dr Gao Bo, Associate Professor, School of Biomedical Sciences, Faculty of Medicine, The Chinese University of Hong Kong. The first authors were Wang Xiaolu, Department of Orthopaedics and Traumatology, School of Clinical Medicine, HKUMed, and Yue Ming, School of Biomedical Sciences, HKUMed. Members of the research team were Dr Prudence Cheung Wing-hang, Wang Xiaojun, Dr Hu Yong, and Professor Keith Luk Dip-kei, Department of Orthopaedics and Traumatology, School of Clinical Medicine, HKUMed; Fan Yanhui, Wu Meicheng, Chen Zheyi and Professor Danny Chan, School of Biomedical Sciences, HKUMed; Professor Tu Wenwei and Dr Wang Xiwei, Department of Paediatrics and Adolescent Medicine, School of Clinical Medicine, HKUMed. Also, Peking Union Medical College Hospital; Texas Scottish Rite Hospital for Children; Centre for Regenerative Medicine and Health, Hong Kong Institute of Science & Innovation, Chinese Academy of Sciences; and RIKEN Center for Integrative Medical Sciences, Japan.
Acknowledgements
The research was supported by the Health and Medical Research Fund (06171406), the Research Impact Fund (R5017-18F), and the Hong Kong Research Grants Council General Research Fund (17114519).
[1]Fong DY, Cheung KM, Wong YW, Wan YY, Lee CF, Lam TP, Cheng JC, Ng BK, Luk KD. A population-based cohort study of 394,401 children followed for 10 years exhibits sustained effectiveness of scoliosis screening. Spine J. 2015 May 1;15(5):825-33. doi: 10.1016/j.spinee.2015.01.019. Epub 2015 Jan 20. PMID: 25615844
Media enquiries
Please contact LKS Faculty of Medicine of The University of Hong Kong by email (medmedia@hku.hk).


Follow HKUMed