![]()
![]()

![]()
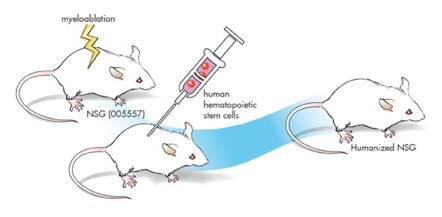
Objective
|
![]()
![]()

Significance of the Platform Technology |
|
Uniqueness of the Platform Technology |
![]()
![]()
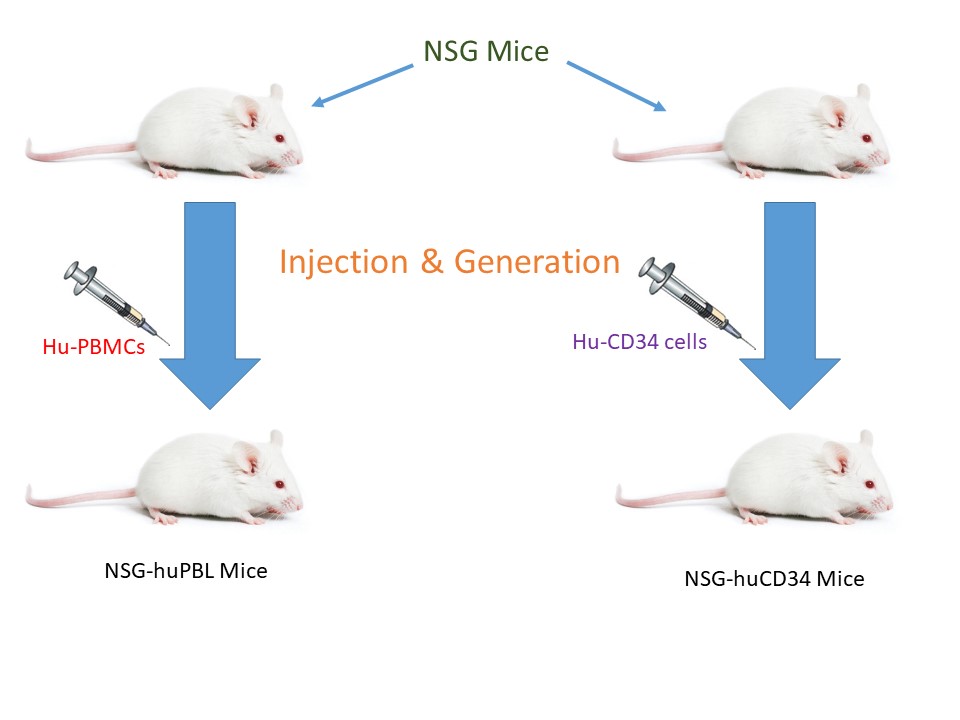
![]()
![]()
Our Publications
(2) Cheung AKL, Kwok HY, Huang Y, Chen M, Mo Y, Wu X, Lam KS, Kong HK, Lau TCK, Zhou J, Li J, Cheng L, Kiat Lee B, Peng Q, Lu X, An M, Wang H, Shang H, Zhou B, Wu H, Xu A, Yuen KY, Chen Z*. Gut-homing Δ42PD1+Vδ2 T cells promote innate mucosal damage via TLR4 during acute HIV type 1 infection. Nat Microbiol. 2017 Oct;2(10):1389-1402.
(3) Wu X, Guo J, Niu M, An M, Liu L, Wang H, Jin X, Zhang Q, Lam KS, Wu T, Wang H, Wang Q, Du Y, Li J, Cheng L, Tang HY, Shang H, Zhang L, Zhou P, Chen Z*. Tandem bispecific neutralizing antibody eliminates HIV-1 infection in humanized mice. Journal of Clinical Investigation. 2018 Apr 23. pii: 96764. doi: 10.1172/JCI96764.
(4) Ling L, Tang X, Huang X, Li J, Wang H, Chen Z. AAV-Vectored Fms-Related Tyrosine Kinase 3 Ligand Inhibits CD34+ Progenitor Cell Engraftment in Humanized Mice. J Neuroimmune Pharmacol. 2018 Dec;13(4):541-550. doi: 10.1007/s11481-018-9819-0. Epub 2018 Oct 30.
(5) Ling L, Wu T, To KKW, Cheung KW, Lui KOL, Niu M, Lam KS, Wang CC, Li J, Wang H, Yuen KY, Chen Z. Vedolizumab-mediated integrin α4β7 blockade does not control HIV-1SF162 rebound after combination antiretroviral therapy interruption in humanized mice. AIDS. 2019 Mar 15;33(4):F1-F12. doi: 10.1097/QAD.0000000000002149.
|