Poster Presentation
The study of HGF/c-met in promoting endothelial progenitor cell migration in the treatment of pulmonary hypertension
Professor An Yong
Children's Hospital of Chongqing Medical University
Abstract
Purpose
Pulmonary hypertension is a group of clinical pathophysiological syndrome, which is characterized by the continuous increase of pulmonary vascular resistance, vascular contraction, vascular remodeling and thrombosis, which is caused by different pathogenesis. Hypoxia-induced pulmonary vascular endothelial injury is a starting factor in the formation of hypoxic pulmonary hypertension. In recent years, cell transplantation has become a new strategy for the treatment of pulmonary hypertension. A large number of experimental studies have confirmed that endothelial progenitor cells (EPCs) can gather in the damaged parts of the vessel and participate in vascular repair and angiogenesis. Therefore, EPCs transplantation therapy is the ideal cell to repair the damaged vascular endothelium and inhibit the formation of pulmonary hypertension from the source. The study found that hepatocyte growth factor (HGF) can modulate vasoactive substances by combining with hepatocyte growth factor receptor (met) to promote the repair of endothelium and reduce pulmonary arterial pressure. HGF can also mediate the EPCs dominance distribution to the injured limb blood vessels to participate in the repair. The research objectives of this experiment include: To investigate the survival of c-met- EPCs in the transplanted body; To study the distribution of c-met- EPCs to damaged target vessels and to obtain the dynamic distribution data of c-met- EPCs in HPH rats; 3. The effect of HGF/c-met axis-mediated EPCs on the contraction and remodeling of injured pulmonary vessels was preliminarily discussed.
Methods
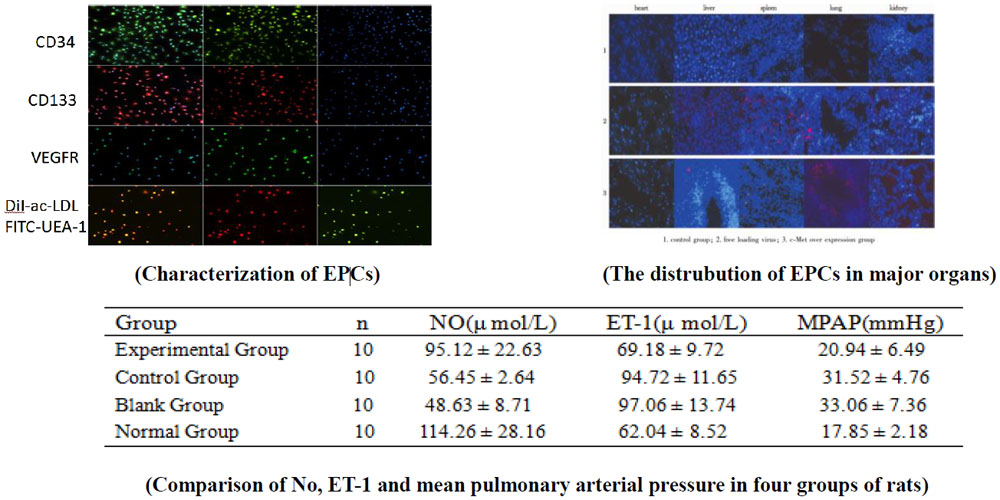
- The rat bone marrow EPCs was isolated by differential wall method. Cell identification: Cell morphological characteristics, immunofluorescence identification of cell surface antigen CD34, CD133 and vascular endothelial growth factor receptor -2 (VEGFR-2), and Dil labeled acetylation of low-density lipoprotein (DIL-AC-LDL) and FITC labeled ulex europaeus agglutinin 1 ( FITC-UEA-1) double fluorescent staining for identification of EPCs.
- Recombinant adenovirus vector mediated met gene transfection EPCs, thus obtaining ad-c-met- EPCs, and then using real-time quantitative PCR detecting system (QPCR) and Western blotting respectively to detect the expression of met in gene and protein level.
- After obtaining ad-c-met- EPCs, using CCK8 to detect met gene has no effect on the proliferative ability of EPCs.
- Using transwell migration system to detect the effects of different concentrations of HGF on ad-c-met-EPCs migration capacity, respectively, with concentrations of 0, 5, 10, 20 and 50ng/ml HGF treated ad-c-met-epcs. Selecting the appropriate HGF (20ng/ml) Treatment of EPCs, Ad-GFP- EPCs and ad-c-met-EPCs, and setting up the PBS control group and the 3-kinase inhibitor LY294002 group (simultaneous addition of 20ng/ml HGF and 10mg/ ml LY294002), through the transwell migration system to detect the migration capacity of each group of cells.
- Distribution of transplanted c-met-epcs in rats
Fluorescence labeled c-met- EPCs was injected into HPH rats through the tail vein, and the data were obtained by liquid scintillation counting, RT-PCR and dynamic MRI, and the distribution of c-met- EPCs was observed. - Effect of HGF/c-met on contraction and remodeling of injured pulmonary vessels
The Model rats with pulmonary hypertension were divided into blank group (saline), experimental group (c-met-EPCS), control group (EPCs) three groups, and then the normal group rats were treated with nitrogen. The changes of the ultrastructure of pulmonary artery were observed by means of the catheter and the average pulmonary artery pressure and electron microscope were detected, and the related factors of oxidative stress were nitrogen monoxide(NO), endothelin-1(ET-1)
Results
- Successfully extracted, cultured and identified rat bone marrow EPCs;c-met gene transfection EPCs;
- C-met gene had no significant effect on the proliferation of EPCs.
- HGF /c-met can improve the migration ability of epcs in vitro.
- HGF /c-met facilitates epcs migration and PI3K/AKT signaling pathways.
- HGF/c-met can benefit from the damage of the pulmonary artery and repair the damaged pulmonary vessels. No content increased and ET-1 content decreased after HGF /c-met transplantation. Average pulmonary arterial pressure decreased.
Conclusions
HGF/c-met can promote EPCs migration and migrate EPCs directed to damaged pulmonary vessels. When using EPCs to treat pulmonary hypertension, gene modification of EPCs by met may improve the migration ability of EPCs. The migration of EPCs to damaged lung tissue, which secretes a large number of HGF, may be a new strategy for treating cardiovascular diseases such as pulmonary hypertension.


