03 August 2022
Researchers from Department of Microbiology, School of Clinical Medicine, Li Ka Shing Faculty of Medicine of The University of Hong Kong (HKUMed) revealed insights into the mechanism of how coronaviruses including SARS-CoV-2, SARS-CoV-1, and MERS-CoV exploit a host protease called ‘cysteine-aspartic protease 6’ (caspase-6) for efficient replication. The findings are peer reviewed and recently accepted for publication in the leading scientific journal Nature [link to publication].
Background
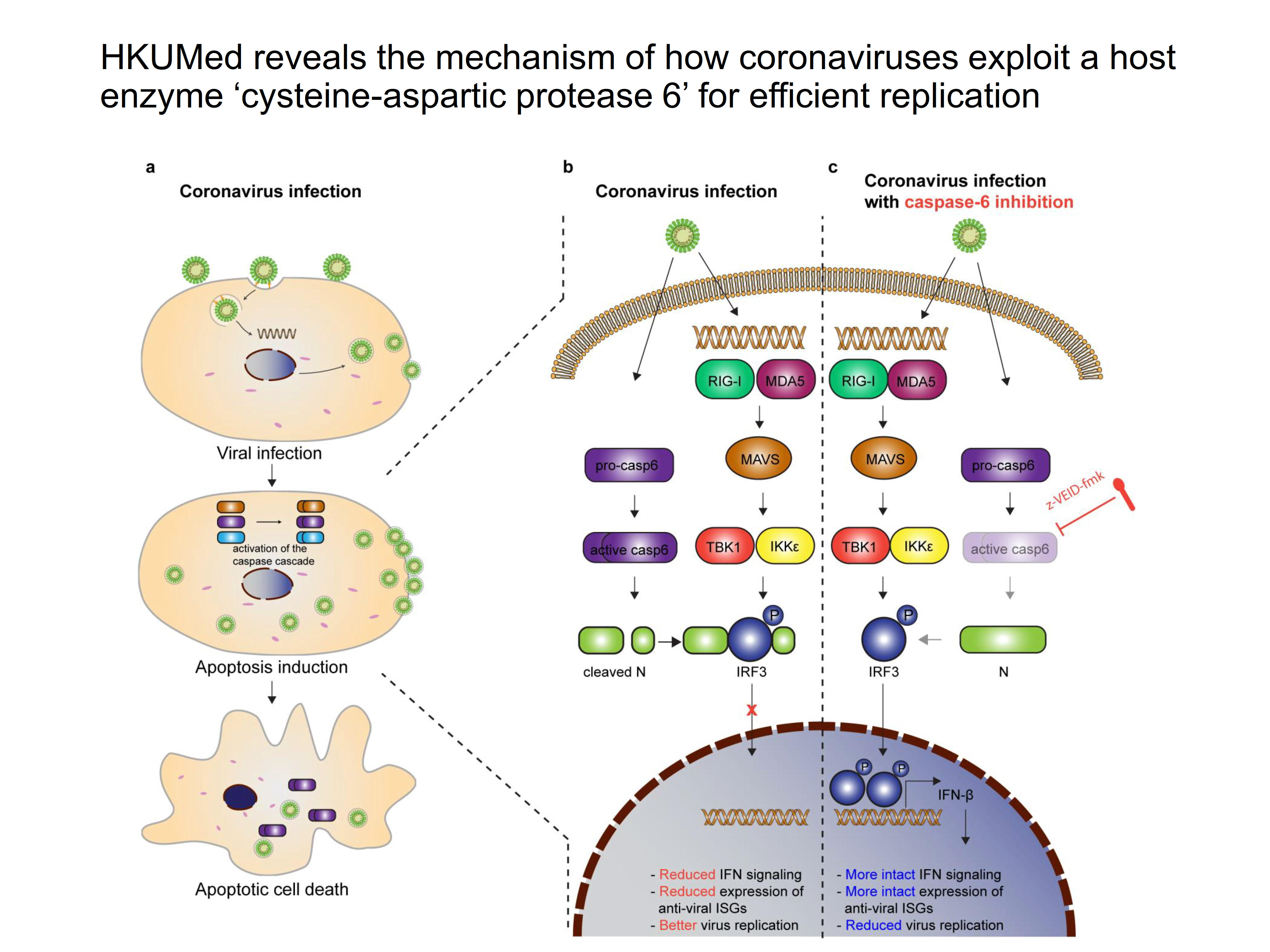
Upon entering the host cells, the ribonucleic acid (RNA) of coronaviruses will trigger the infected cells to secrete interferons that can inhibit virus replication within the infected cells, and reduce the risk of infection among other uninfected cells. Meanwhile, the host cells will also undergo apoptosis process, a programmed cell death process which will eliminate cells from becoming factories of viral replication. Hence the interferon reaction and apoptosis process are the most important anti-virus mechanisms in human and animal cells.
Apoptosis is executed by cysteine-aspartic protease in human and animal cells, by which infected cells are eradicated to inhibit virus replication. The HKUMed team investigated the impact of caspase-6 on coronavirus replication in a series of cell line, human ex vivo lung tissue, human intestinal organoids, and animal models. The team found that the chemical inhibition or gene depletion of caspase-6 significantly reduced coronavirus replication, while overexpressing caspase-6 efficiently promoted coronavirus replication.
Research methodology and findings
In a mouse model, chemical inhibitors of caspase-6 dramatically limited mouse-adapted MERS-CoV (MERS-CoVMA) replication and significantly improved the survival of mice from 33.3% to 80%. Moreover, MERS-CoVMA replication and MERS-CoVMA-induced lung damage were markedly reduced in the lungs of caspase-6 knockout mice. Similarly, in a hamster model, the specific chemical inhibitor against caspase-6 reduced SARS-CoV-2 replication and the associated inflammatory lung damage.
But how does the coronavirus harness caspase-6 to its benefit? Note that the interferon response is the most important and immediate antiviral defence of host cells. Our further investigations revealed that caspase-6 cleaved the coronavirus nucleocapsid (N) proteins, generating N fragments that interact with the host ‘interferon regulatory factor 3 (IRF3)’ and stop it from entering the cell nucleus to initiate the interferon response. Thus, the viral N fragments serve as interferon antagonists, which facilitate virus replication.
Significance of the study
The study shows how coronaviruses have evolved to become very successful pathogens. We reveal a novel mechanism of how coronaviruses overcome the antiviral defence of interferon response by the host cells through the exploitation of a new class of host protease, caspase-6, which is originally used by the host for executing cell apoptosis as a defence against virus infection, for its own purpose of getting better viral replication. These results further suggest that drugs can be designed against caspase-6, which can become a potential target of intervention for the antiviral treatment of all known human coronavirus infections.
About the research team
The research is led by Dr Chu Hin, Assistant Professor; Dr Jasper Chan Fuk-woo, Clinical Associate Professor and Professor Yuen Kwok-yung, Henry Fok Professor in Infectious Diseases, Chair Professor of Department of Microbiology, School of Clinical Medicine, HKUMed, Director of the State Key Laboratory of Emerging Infectious Diseases and Academician of the Chinese Academy of Engineering.
Media enquiries
Please contact LKS Faculty of Medicine of The University of Hong Kong by email (medmedia@hku.hk).

Follow HKUMed