02 March 2021
A research team at the School of Biomedical Sciences, LKS Faculty of Medicine, The University of Hong Kong (HKUMed) identified a stable Spindlin1/C11orf84 protein complex preferentially recognises a non-canonical bivalent histone H3K4me3K9me3 mark and promotes rRNA transcription. The study has opened a new therapeutic avenue to design inhibitors targeting Spindlin1/C11orf84 complex for cancer therapy. The finding has been published in a leading academic journal, Nature Communications. [Link to the publication]
Background
Cancer is a leading cause of death worldwide. As a hallmark of cancer, abnormal cell growth and proliferation is sustained by increased ribosome biogenesis. Ribosomal RNA (rRNA) transcription, as the first key step of ribosome biogenesis, is tightly regulated by multi-layered epigenetic mechanisms. Spindlin1 is a well-established epigenetic reader that plays a critical role in activating rRNA transcription.
Research findings
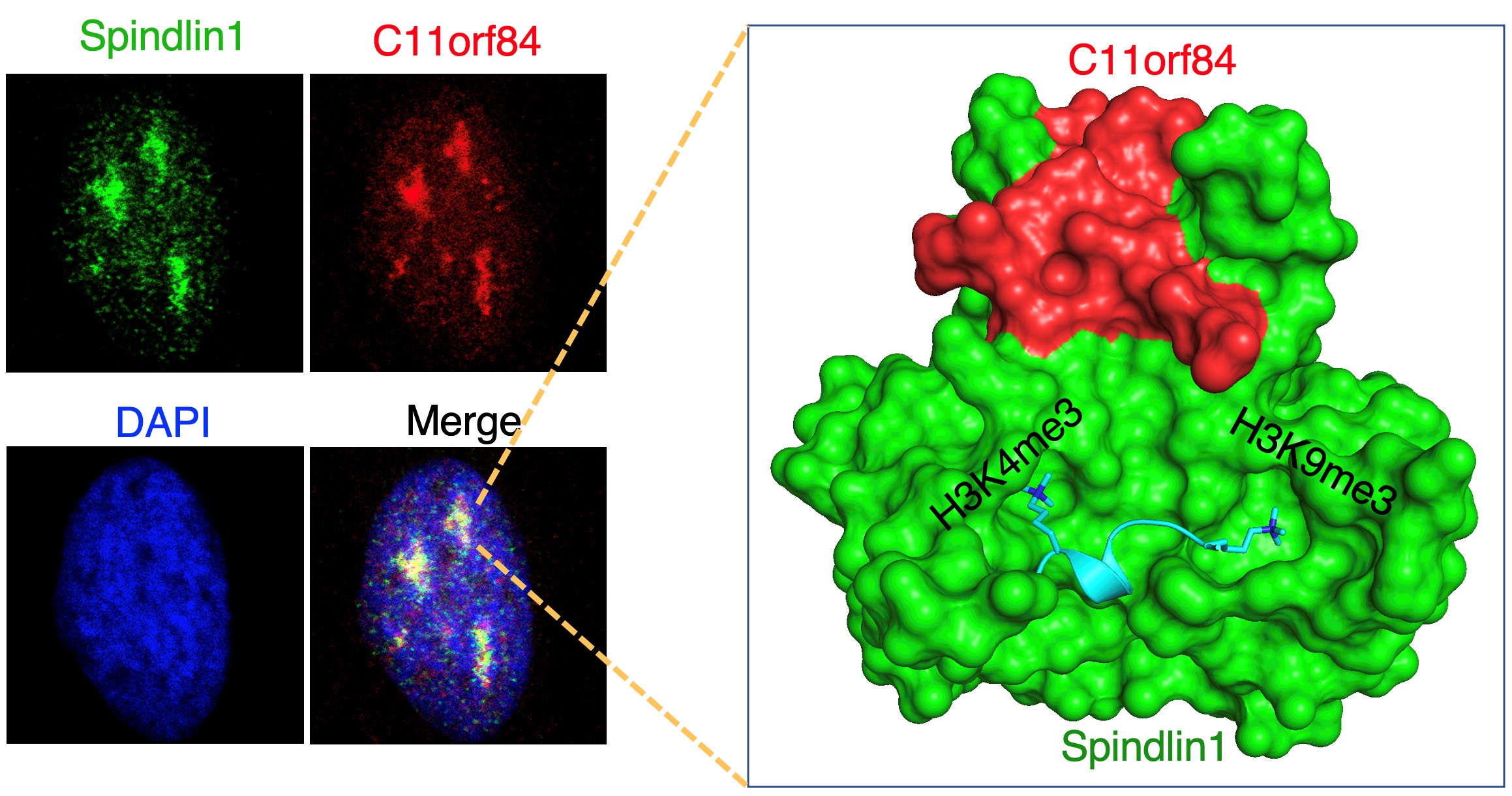
The researchers firstly identified that C11orf84 is a bona fide binding partner of Spindlin1. C11orf84 co-localises with Spindlin1 in the nucleolus and stabilises Spindlin1. They then found this protein complex has the preference to recognise non-canonical bivalent H3K4me3K9me3 mark, and shows the strongest binding to methylated histones among the known histone reader proteins.
The researchers further provided the detailed molecular mechanism of this novel epigenetic recognition by determining the high-resolution crystal structure of Spindlin1/C11orf84/H3K4me3K9me3 ternary complex. Furthermore, the researchers showed that overexpression of Spindlin1 could outcompete HP1 protein binding at poised rRNA gene marked by H3K4me3K9me3 to activate rRNA transcription. Consistently, both Spindlin1 and C11orf84 are overexpressed in multiple types of tumour cells and tissues, knockdown of C11orf84 or Spindlin1 slows down tumour cell growth.
Significance of the study
Dr Qian Chengmin, Associate Professor at the School of Biomedical Science, HKUMed, who coordinated this study, said, “This study reveals a previously unappreciated mechanism of bivalent H3K4me3K9me3 recognition by Spindlin1/C11orf84 complex. It not only provides a rational molecular explanation of Spindlin1 in rRNA transcription activation, but also builds a solid foundation for future exploring the role of Spindlin1/C11orf84 in early embryonic development.”
“Given that C11orf84 and Spindlin1 are often overexpressed in many types of human cancers and involved in tumor cell growth, migration and invasion, to design specific inhibitors to target these two proteins will provide a new therapeutic strategy for cancer therapy,” Dr Du Yongming, Post-doctoral Fellow at the School of Biomedical Science, HKUMed, as well as the first author of the study added.
About the research team
The study was conducted by Dr Qian Chengmin’s research group at School of Biomedical Sciences, Li Ka Shing Faculty of Medicine, The University of Hong Kong (HKUMed). Professor Zhou Ming-Ming, Department of Pharmacological Sciences, Icahn School of Medicine at Mount Sinai, New York and Dr Wang Xin’s group from Department of Biomedical Sciences, The City University of Hong Kong also contributed to this study. Dr Qian’s research group has interest in unravelling molecular mechanisms of genome instability caused by epigenetic changes and DNA damage. This study is mainly funded by General Research Fund (17127917, 17160016 and 17129218) of the University Grants Committee, Government of the Hong Kong Special Administrative Region.
Media enquiries
Please contact LKS Faculty of Medicine of The University of Hong Kong by email (medmedia@hku.hk).


Follow HKUMed